
Lithium-ion batteries (LIBs) have dominated the market of portable electronics and electric vehicles due to their high energy density and long-term cyclability, and are moving forward to scale energy storage applications such as regulating the output of electricity generated by sustainable energy. Nevertheless, the current electrochemistry of LIBs based on Li-ion interaction/de-interaction between graphite anode and oxide cathode is suffering from intrinsic limitations of energy density, scarce natural resource (Li, Co, Ni etc.), and high energy consumption/CO2 emission involved in the production of electrodes. Organic redox compounds, especially conjugated carbonyl compounds that have been studied since 1969, are reviving in the recent years due to the advantages of high capacity, abundant resources, and structural designability. Moreover, the electrochemical redox mechanism of organic carbonyl electrode materials mainly based on charge compensation enables the battery applications with versatile charge carriers (Li+, Na+, H+ etc.). The key challenges of organic carbonyl electrode materials are their high solubility in electrolyte during cycles and poor electronic conductivity, leading to fast capacity decay and inferior rate performance, respectively. This report focuses on the redox chemistry, structure-performance relationship, and applications of organic carbonyl electrode materials for Li and Na batteries. We developed several strategies from the aspects of molecular design (electrode level) and electrolyte optimization (electrolyte level) to solve the issues of organic carbonyl electrodes and construct high-performance Li/Na batteries. With elaborate design, organic carbonyl electrode materials have demonstrated promising interest for large-scale electrochemical energy storage in the foreseeable future.
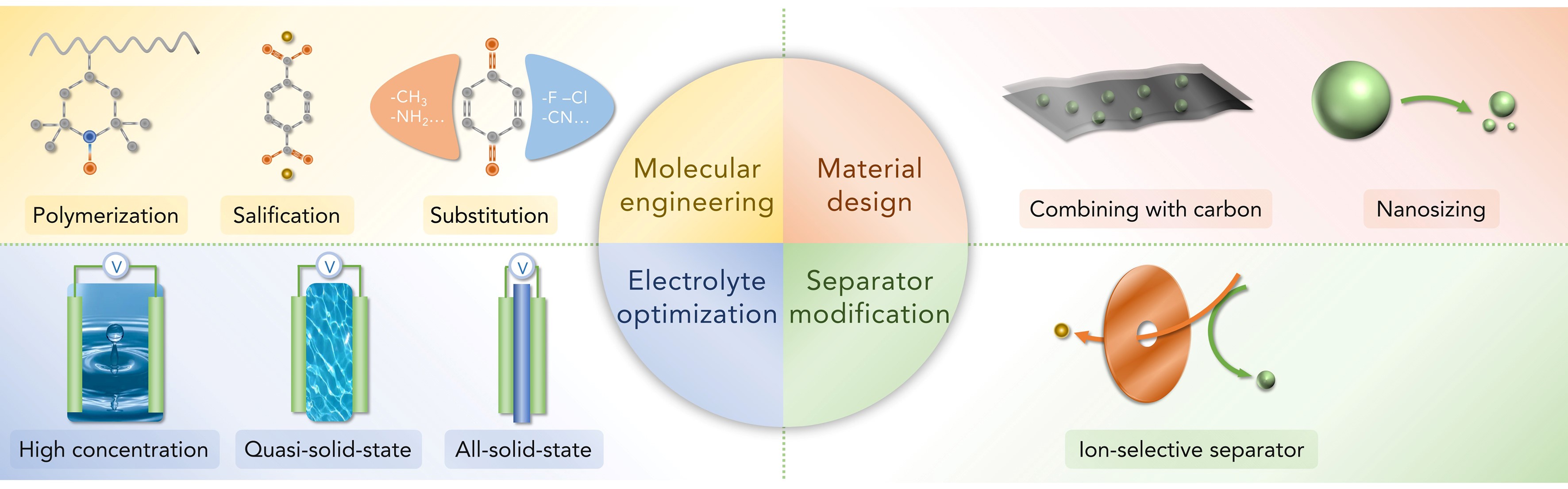 Figure 1: Proposed strategies toward improving the electrochemical performance of organic carbonyl electrodes for Li/Na batteries.
Figure 1: Proposed strategies toward improving the electrochemical performance of organic carbonyl electrodes for Li/Na batteries.
References and selected publications
[1] D. L. Williams, J. J. Byrne, J. S. Driscoll. J. Electrochem. Soc. 1969, 116, 2–4.
[2] H. Chen, M. Armand, G. Demailly, F. Dolhem, P. Poizot, J.-M. Tarascon. ChemSusChem 2008, 1, 348–355.
[3] Y. Liang, Z. Tao, J. Chen. Adv. Energy Mater. 2012, 2, 742–769.
[4] Y. Liang, P. Zhang, J. Chen. Chem. Sci. 2013, 4, 1330–1337.
[5] Y. Liang, P. Zhang, S. Yang, Z. Tao, J. Chen. Adv. Energy Mater. 2013, 3, 600–605.
[6] W. Huang, Z. Zhu, L. Wang, S. Wang, H. Li, Z. Tao, J. Shi, L. Guan, J. Chen. Angew. Chem. Int. Ed. 2013, 52, 9162–9166.
[7] Z. Zhu, M. Hong, D. Guo, J. Shi, Z. Tao, J. Chen. J. Am. Chem. Soc. 2014, 136, 16461−16464.
[8] S. Wang, L. Wang, Z. Zhu, Z. Hu, Q. Zhao, J. Chen. Angew. Chem. Int. Ed. 2014, 53, 5892–5896.
[9] Z. Luo, L. Liu, Q. Zhao, F. Li, J. Chen. Angew. Chem. Int. Ed. 2017, 56, 12561–12565.
[10] R. Shi, L. Liu, Y. Lu, C. Wang, Y. Li, L. Li, Z. Yan, J. Chen. Nature Commun. 2020, 11, 178.
[11] Y. Lu, J. Chen. Nature Rev. Chem. 2020, 4, 127–142.
[12] Y. Lu, Y. Cai, Q. Zhang, J. Chen. Adv. Mater. 2022, 34, 2104150